Perfect Single-Crystal Alumina Microspheres
Weiyou Yang, Huatao Wang, Xiaomin Cheng, Zhipeng Xie, and Linan An
We report the synthesis of perfect single-crystal Al2O3 microspheres via direct thermal decomposition of aluminum isopropoxide in air without any template or catalyst. The obtained microspheres were characterized using scanning electron microscopy, X-ray diffraction, transmission electron microscopy, and selective area electron diffraction. The results suggested that the formation of the spheres can be attributed to a transition from polycrystalline to single-crystalline symmetry during high-temperature annealing. The obtained microspheres could be useful for many applications such as lubricants and polishing mediums.
I. Introduction
Alumina powder is one of the most important materials with widespread applications, including starting materials for advanced ceramics, catalysts/catalyst carriers, polishing mediums, etc. For many of these applications, synthesis of the powder with a spherical shape is critical. Alumina powder was synthesized by means of a variety of methods, including transferred arc process, flame spray pyrolysis, wet chemistry and calcining process, laser ablation condensation, chemical precipitation, spray pyrolysis, and mechanochemical/thermal processing. However, the powders synthesized by these techniques are irregularly faceted. Morphologies of single-crystal micro-/nanoparticles are determined by their crystalline symmetries and synthesis processes. Given its crystalline structure, only two kinds of shapes could be obtained for alumina powder: a kinetically controlled regular shape or a thermodynamically controlled faceted shape. Synthesis of single-crystal Al2O3 particles of spherical shape is a significant technical challenge because spherical single-crystal particles must have energetically unfavorable high-index surfaces.
Here, we report the large-scale synthesis of single-crystal alumina microspheres by direct thermal decomposition of an aluminum metallorganic compound. Detailed studies on structural evolution during annealing show that the formation of the spheres is due to a transition from polycrystalline spheres to single-crystalline ones during high-temperature annealing. The spherical alumina powder is promising for many important applications.
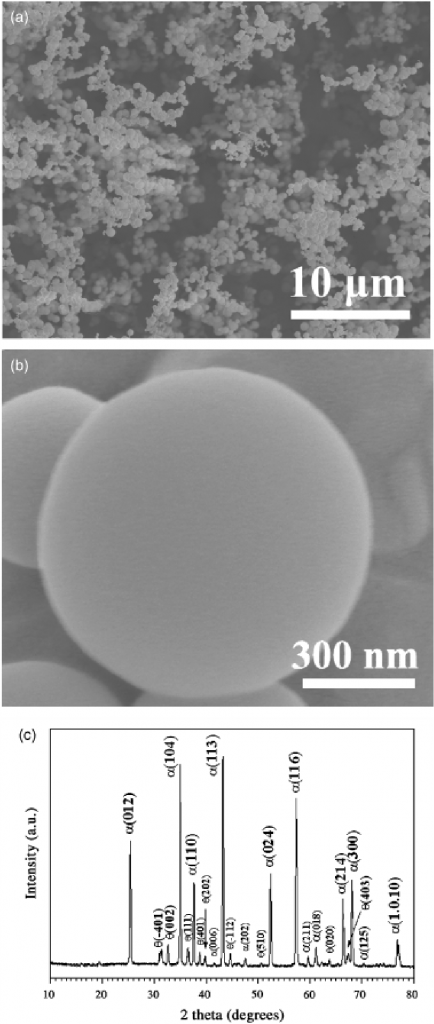
Fig.1. (a) A typical low-magnification scanning electron microscopy (SEM) image of the synthesized product. (b) A high-magnification SEM image of an individual particle. (c) An X-ray diffraction pattern of the synthesized product.
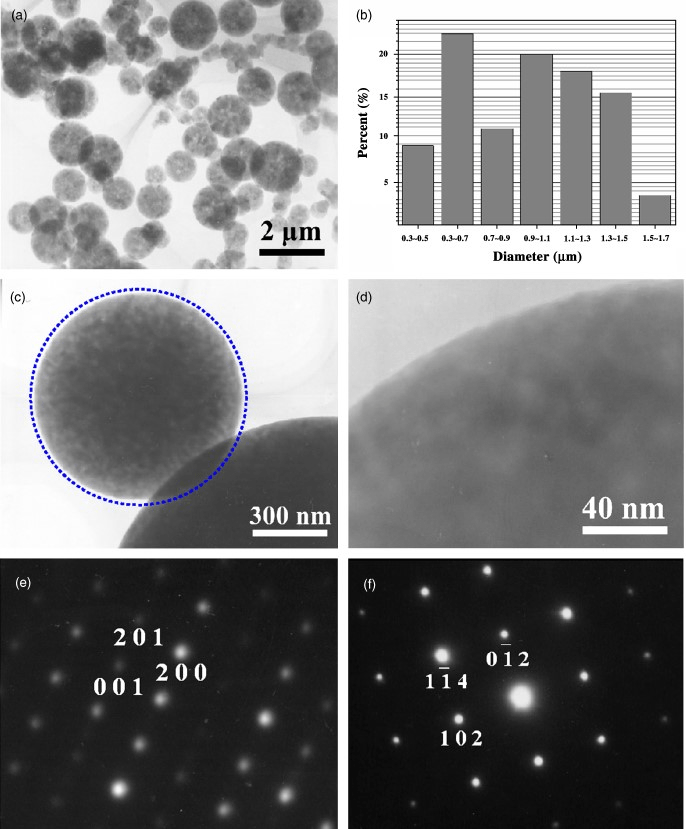
Fig.2. (a) A typical low-magnification TEM image of Al2O3 microspheres. (b) Size distribution of the microspheres synthesized at 13501C for 1 h. (c) A high-magnification TEM image of an individual Al2O3 microsphere showing the perfect spherical morphology. (d) A high-magnification TEM image revealing the smooth surface of the synthesized particles. (e) and (f) Typical selected-area electron diffraction patterns recorded from nanosized and microsized particles, showing that both of them are single crystals in nature and the nanoscale particle is γ-Al2O3 whereas the microsized particle is α-Al2O3.
II. Experimental Procedure
Al2O3 microspheres were synthesized by thermal decomposition of aluminum isopropoxide (AIP) at 13501C for 1 h in air without any template or catalyst. The powder was placed in a high-purity Al2O3 crucible. Thermal decomposition was carried out in a conventional tube furnace with open ends.
The obtained products were characterized using fieldemission scanning electron microscopy (SEM, JSM-6301F, JEOL, Tokyo, Japan), X-ray diffraction (XRD, Automated D/Max-RB, Rigaku, Japan) with CuKa radiation (l51.54178 A˚ ), and transmission electron microscopy (HRTEM, JEOL2011) equipped with an energy-dispersive spectrum (EDS).
III. Results and Discussion
The obtained product was first observed by SEM. Figure 1(a) is a typical low-magnification SEM image showing that the AIP was completely converted to loosely packed particles. Closer observation of the particles reveals that they exhibit a perfect spherical shape with a smooth surface (Fig. 1(b)). The XRD study (Fig. 1(c)) reveals that there are two phases in the obtained particles: a major a-Al2O3 phase and a small amount of monoclinic y-Al2O3 phase. The formation of a-Al2O3 as the major phase is due to the high temperature (13501C) used in the current experiment, because transition phases such as k-, d-, and g-Al2O3 were found to transform into the thermodynamically stable a-Al2O3 when the temperatures were higher than ~1200℃.14,15
Further characterization of the spherical particles was carried out using TEM. Figure 2(a) is a typical low-magnification TEM image of the particles, showing that in addition to the relatively large particles there is a small amount of very tiny particles of B100 nm size. Typical EDS obtained from a single microsphere under TEM revealed that the atomic ratio of Al to O, within the experimental limit, is close to 2:3. The particle size distribution of the large particles was measured for over 200 particles from the low-magnification TEM images, and plotted in Fig. 2(b). Most of the particles exhibit a perfect spherical shape, which can be seen more clearly from the high-magnification TEM image (Fig. 2(c)), where the artificially drawn standard circle matches the shape of the microsphere very well. The observation of the particle at even higher magnifications reveals that the particles exhibit perfect smooth surfaces (Fig. 2(d)). Figure 2(e) and (f) are typical selected-area electron diffraction (SAED) patterns recorded from a tiny particle and a relatively large particle, respectively. Analysis of these patterns reveals that the tiny particles (Fig. 3(e)) have monoclinic y-Al2O3 structure with lattice parameters a51.181 nm, b50.2906 nm, and c50.5625 nm, whereas the large particles (Fig. 3(f)) are hexagonal a-Al2O3 with a50.4758 nm and c51.2992 nm. The patterns also reveal that all the particles are single crystals in nature, regardless of their size.
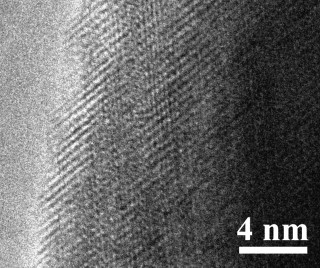
Fig.3. A typical high-resolution TEM image recorded from an individual Al2O3 microsphere, revealing no amorphous layer on its surface.
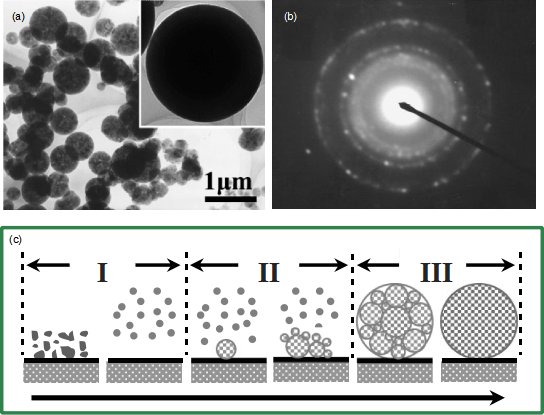
Recently, single-crystal ceria nanospheres were synthesized by Feng et al.16 They attributed the formation of the spherical shape to the existence of an amorphous layer on the surface of the nanospheres, which overcame the high energy associated with high-index crystal planes by minimizing the surface energy. To check whether the same mechanism is applicable in the current case, the microspheres were observed using high-resolution TEM (Fig. 3). The result reveals that the surfaces of the microspheres are clean without any amorphous layers, suggesting that the growth mechanism reported in Feng et al.16 cannot be used to explain the formation of the microspheres here. To understand the process of formation of the Al2O3 microspheres, the AIP were decomposed at different temperatures. Figure 4 shows the particles obtained at 12501C for 1 h. The particles obtained at this stage already have a close-to-perfect spherical shape. However, an SAED pattern (Fig. 4(b)) suggests that the particles obtained at this lower temperature are polycrystalline instead of single crystalline. This result suggests that the single-crystal Al2O3 microspheres were formed by converting the polycrystalline microspheres at ahigher temperature.
Based on these results, we propose a three-stage growth process to account for the formation of the single-crystal Al2O3 microspheres, as illustrated in Fig. 4(c). In stage I, AIP reacts with O2 in air to form the gas phase of Al2O at a temperature of 11001C, accompanied by the gaseous by-products CO2 and H2O via reaction (1).15 The Al2O further reacted with O2 to form Al2O3 via reaction (2),17 and/or the Al2O decomposed into Al2O3 via reaction (3).15 The Al formed in reaction (3) can be further oxidized to Al2O3. The reactions involved in the process are

In stage II, the Al2O3 molecules were condensed to form Al2O3 particles via a nucleation and growth process. Because of the relatively low processing temperature and high mass flow rate, the condensation was a multiple-nucleation process, leading to the formation of polycrystalline particles (Figs. 4(a) and (b)). Because of the relatively high processing temperature, the individual grains within each polycrystalline Al2O3 particle should have a spherical shape to minimize surface energy. In stage III, the polycrystalline particles were converted to singlecrystalline ones via grain growth from one grain.

Fig.4. (a) A typical TEM image of the Al2O3 microspheres synthesized at 12501C for 1 h; the inset is a TEM image of an individual microsphere showing its perfect morphology. (b) The corresponding selected-area electron diffraction pattern indicates the sphere is polycrystalline in nature. (c) Schematic showing the proposed growth process of the single-crystal Al2O3 microspheres.
IV. Summary
In summary, single-crystal Al2O3 microspheres are synthesized by direct thermal decomposition of AIP in air without any template or catalyst. SEM and TEM observations reveal that the microspheres possess close-to-perfect spherical shape. Study on the pyrolysis products obtained at different temperatures suggests that the formation of the close-to-perfect spherical shape is due to a transition from polycrystalline to single-crystalline symmetry during annealing. The results suggest a simple way of synthesizing the single-crystal Al2O3 microspheres, which could find widespread applications such as lubricants and polishing mediums.